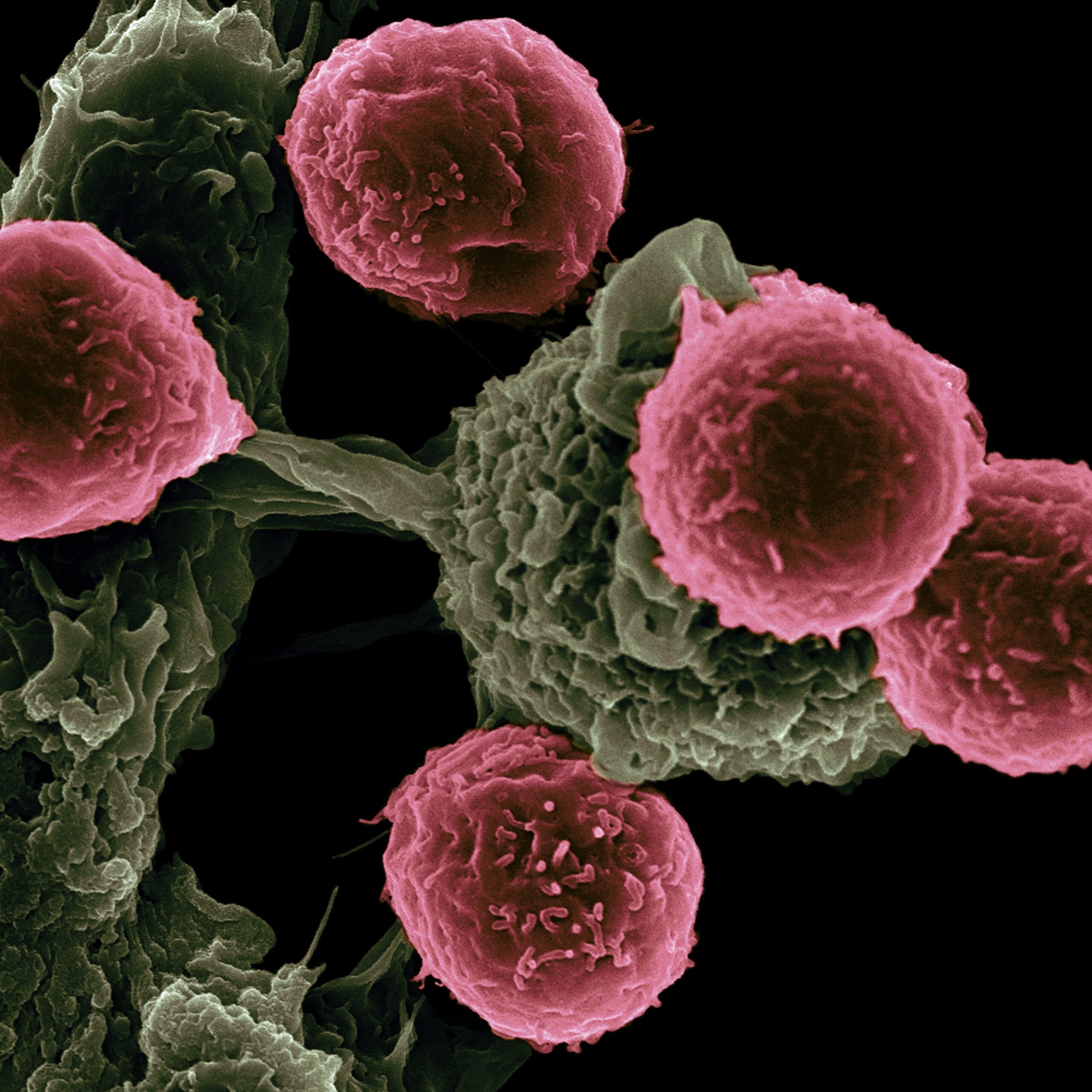
Abcon Therapeutics, Inc. is dedicated to the development of first-in-class therapeutics for treating cancer and autoimmunity. The Company’s first product, ATX006, is a fully humanized anti-CD6 monoclonal antibody. ATX006 binds to CD6, an established T cell marker, at pM affinity and is rapidly internalized after its binding to CD6 on the cell surface. The antibody has been shown to be effective in treating multiple models of solid tumors and autoimmune diseases. The Company’s second product, ATX101, utilizes ATX006 as part of an antibody-drug conjugate (ADC) that targets CD6. ATX101 has been highly effective in treating preclinical models of T-cell lymphoma (TCL), demonstrating tumor shrinkage and inhibition of tumor metastasis. ATX101 also selectively eliminates activated proliferating non-malignant T cells and showed efficacy in treating preclinical models of autoimmune uveitis and graft-versus-host disease, in both of which activated T cells cause the disease.
The National Cancer Institute’s NExT program is partnering with Abcon to carry out the non-GMP and GMP manufacturing and preclinical toxicology, pharmacokinetic and pharmacodynamic work required to advance ATX101 into human clinical trials. Based on the restricted expression profile of CD6, the rapid and complete internalization of ATX101 and the known efficacy of other FDA-approved ADCs targeting different proteins but using the same linker and payload technology, ATX101 is expected to be a safe and effective treatment for aggressive TCL.